News & Stories
See the latest news about CMT drug development and read stories from the CMT community that highlight why we must deliver treatments and cures during our lifetime.

What to Expect in CMT Research During 2021: 5 Questions with Chief Scientific Officer Keith Fargo
What progress can patients and families expect to see in CMT research during 2021? What is the most promising research on the horizon? The CMT Research Foundation’s Chief Scientific Officer Keith Fargo, Ph.D., sat down with us to answer your most pressing questions about CMT research in the year ahead.
How to Measure Progress in CMT Research: 8 Signs Research is Working
It costs more than $2.6 billion to develop an approved prescription medicine and typically takes between 10 to 15 years to get a drug to clinical trials. With no treatments or cures currently available for diseases like CMT, it’s easy to question how donations to support scientific research make a difference. These 8 signs let you know when research is working.
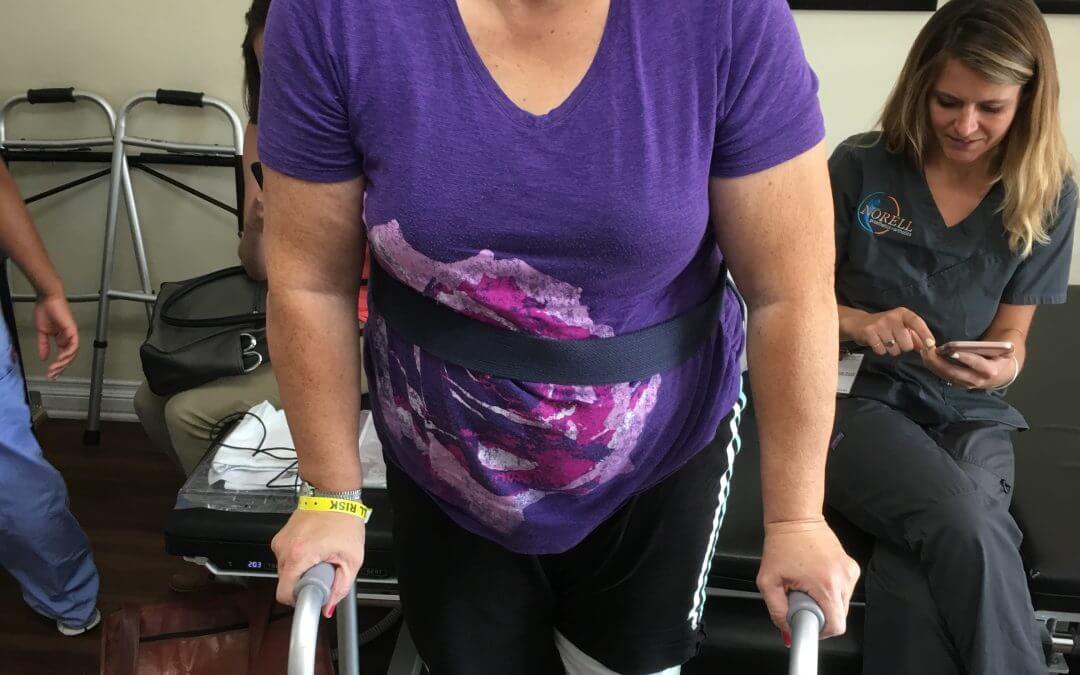
Mom with CMT Loses Her Leg but Gains Strength for Her Daughter
My doctor told me it was the worst case of CMT he had ever seen. We reviewed my options: A fusion with no end to my pain, or amputation. Here’s what I chose and what I’m doing to change things for my daughter.
After 10 Surgeries in 10 Years to Address Deformities from CMT, This Young Woman is Fighting Back
Surgical recovery takes up to 12 weeks and requires me to take a leave of absence from my job, lay with my toes above my nose, spend most of the day alone, and give up my independence. I’m unable to shower on my own, walk without crutches, and have become very socially isolated, especially during COVID-19 quarantine. Given that I’ve had so many surgeries in such a short period of time, it’s difficult to maintain steady employment, let alone a full-time career. All of that is on hold due to my CMT.

Hope Alone Won’t Save My Hands, But a Treatment Will
I heard a loud pop. Then came the sudden swelling. All I’d been doing was walking down the sidewalk when my left leg snapped. At first, I thought it was just sprained. I kept limping on it for two weeks, until a doctor confirmed it was broken. Doctors gave me three options: Live with the pain and wear a removable cast for the rest of my life, undergo another surgery that probably won’t work, or amputate a portion of my leg. I chose amputation. Read more about Joe’s journey and what he’s doing to give people with CMT more than hope.

AcuraStem Scientists Demonstrate Positive Early Results for CMT2A Treatment
The CMT Research Foundation is currently funding a research project led by AcuraStem aimed at producing effective treatments for CMT2A. Using stem cells derived from adult patients, AcuraStem scientists have tested thousands of compounds for their ability to promote...

CMT Research Foundation Project Shows Progress in a Gene Therapy Approach to Treat CMT1A
The CMT Research Foundation is currently funding a research project led by Dr. Kleopas Kleopa and his team at the Cyprus Institute of Neurology & Genetics to study a gene therapy approach to lower levels of PMP22, the gene that causes CMT1A. While no one can...

DTx Pharma Shows Continued Progress in Reducing PMP22 Levels in Animal Models of CMT1A
The CMT Research Foundation is currently funding a research project led by DTx Pharma to design genetic therapies for Charcot-Marie-Tooth disease (CMT). By attaching the genetic sequences to molecules called long chain fatty acids, they allow the therapies to target...
One Million Steps to the Holidays for CMT
By: Gary Donaldson, community manager, CMT Research Foundation As I approach my one-year anniversary as the community manager for the CMT Research Foundation, I’ve thought a lot about how I should mark this occasion. I decided the best way to celebrate is by facing...

How We Get from Today to Approved CMT Treatments: An Interview with FDA Director Dr. Peter Marks
The CMT Research Foundation is asking and answering the most pressing questions patients have about the need for treatments and cures. In episode 3, CMT Research Foundation CEO Susan Ruediger interviews the FDA’s Dr. Peter Marks to discuss gene therapies as a potential treatment option for CMT, what the approval process looks like and how patients can expedite it.
NEWSLETTER SIGNUP
Stay up to date on new CMT research, treatments and clinical trials
Address
4062 Peachtree Road
Suite A209
Atlanta, GA 30319
Phone Number
404.806.7180
Media Inquiries
© 2024 CMT Research Foundation | Privacy Policy
