The CMT Research Foundation is proud to announce our latest project, a partnership between AcuraStem, a patient-based drug discovery platform company, and Dr. Alessandra Bolino, a renowned expert in CMT and the head of the Human Inherited Neuropathies Unit at the prestigious research hospital Ospedale San Raffaele in Milan, Italy. The CMT Research Foundation, AcuraStem and Dr. Bolino have agreed to work together to test the most promising of AcuraStem’s new compounds in Dr. Bolino’s CMT4B1 models: AcuraStem will provide the drug candidate, Dr. Bolino will conduct the research and the CMT Research Foundation will fund the project. Dr. Bolino and AcuraStem were introduced by the CMT Research Foundation.
Charcot-Marie-Tooth disease (CMT) is one of the most common inherited neurological disorders, affecting approximately 3 million people across the globe. Despite this, there are as yet no treatments or cures. CMT4B1 is a particularly severe and debilitating form of the disease, with the first symptoms appearing in childhood. The disease is characterized by progressive weakening and loss of muscles, frequently leading to wheelchair use, and sometimes even leading to death from weakening of the muscles that control breathing.
CMT4B1 is caused by a mutation in the gene MTMR2. This gene codes for a protein that is responsible of a small but critical step in regulating the formation of the myelin sheath, breaking down a fatty molecule with the unusual name PI(3,5)P2. When the MTMR2 gene is mutated, this fatty molecule is present in higher levels than it should be because it is no longer being broken down efficiently. This extra PI(3,5)P2 changes the shape of the myelin sheath, leading to abnormal structures called outfoldings. These outfoldings eventually interfere with the normal functioning of the nerve, causing it to degenerate, which leads to the symptoms of CMT4B1.
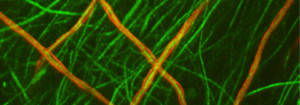
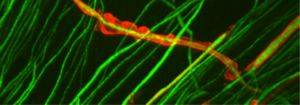
The upper photograph shows peripheral nerve axons in green, some of which are surrounded by myelin in red. Note the neat and regular appearance of the myelin sheath, which is in close contact with the axons. In contrast, the lower photograph shows cells with mutated MTMR2 (CMT4B1 cells). Note the loose, billowy outfoldings of myelin.
Dr. Bolino and the scientists at AcuraStem believe that one way to reduce the amount of PI(3,5)P2 in the peripheral nerves is simply to not make as much of it in the first place. This is a little bit like turning down the faucet when the shower drain is slow to prevent the water from overflowing onto the floor. PI(3,5)P2 is normally produced by an enzyme called PIKfyve, and they hypothesize that slowing down the activity of PIKfyve could prevent the buildup of PI(3,5)P2 and the resulting CMT4B1 disease.
Dr. Bolino has previously shown that treating CMT4B1 cells with a PIKfyve inhibitor prevents the appearance of outfoldings. However, the PIKfyve inhibitor she tested breaks down rapidly in the body and therefore is probably not a good drug candidate. The scientists at AcuraStem have developed a number of new PIKfyve inhibitors with chemical properties that make them last longer, potentially having a therapeutic effect.
The research team in Milan led by Dr. Bolino will test the most promising of AcuraStem’s novel PIKfyve inhibitors in a two-stage project. In the first stage, the researchers will be looking for something that researchers call target engagement – does the drug do what it is supposed to do at a molecular level, that is, does it reduce the amount of PI(3,5)P2 and prevent outfoldings in the myelin sheath? This stage will involve both cellular models and rodent models. In the second stage, the researchers will be examining efficacy – does the drug prevent rodents without functional MTMR2 genes from developing symptoms of CMT4B1? Rodents will be treated daily with the drug (or a placebo) from three weeks of age to 5 months of age, which roughly translates to early childhood and mature adulthood in humans. The researchers will then test the rodents’ nerve functions and structure to determine whether the treatment prevented disease. The full project is expected to take approximately two years to complete. While this particular project is focused on models of CMT4B1, in principle the approach could also be used in other forms of CMT that are characterized by myelin outfoldings, such as CMT4B2, CMT4B3, CMT4C, and CMT4H.
After years of working on this particularly devastating form of CMT, Dr. Bolino is pleased to be testing a compound that may be first to market for those affected.
“After laying the scientific foundation for this kind of drug testing in CMT4B1, it is fantastic to be able to work with a drug candidate like this. Inhibiting PIKfyve has a profound effect on the myelin sheath in our models, and we can’t wait to put this novel PIKfyve inhibitor to the test,” Bolino said.
Sam Alworth, CEO of AcuraStem, agreed. “We are just delighted that Dr. Bolino will be testing our new compound in her CMT4B1 models. Patients are at the heart of everything we do at AcuraStem, and we are hopeful that our drug candidate can prevent kids with MTMR2 mutations from ever experiencing the devastation of CMT4B1.”
The CMT Research Foundation is a nonprofit focused solely on delivering treatments and cures for Charcot-Marie-Tooth disease. We are an organization founded by people with CMT, funded by people with CMT, and led by people with CMT. Our commitment is complete.
