News & Stories
See the latest news about CMT drug development and read stories from the CMT community that highlight why we must deliver treatments and cures during our lifetime.

Update on NMD Pharma Clinical Trial
NMD Pharma, a clinical-stage biotech company dedicated to developing novel and improved treatments for patients living with neuromuscular diseases, has announced that it has dosed the first Charcot-Marie-Tooth disease patient in its Phase 2a clinical trial of NMD670...
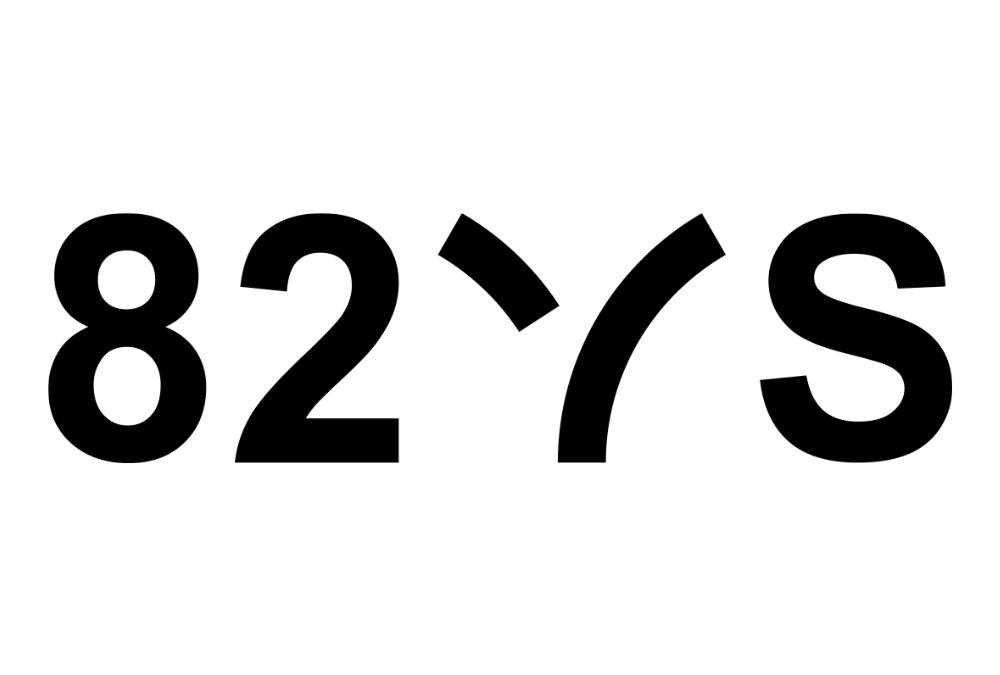
CMT Research Foundation Funds Project with Alloy Therapeutics’ 82VS to Test Novel RNA Therapeutics for Charcot-Marie-Tooth Disease
The CMT Research Foundation has invested over $500,000 in a program led by 82VS, the venture studio of Alloy Therapeutics, Inc. to discover and develop novel Antibody Oligonucleotide Conjugate (AOC) therapeutics for CMT. Oligonucleotide therapeutics offer promise for...

Actio Biosciences Receives Both Orphan Drug and Rare Pediatric Disease Designations From the FDA For Treatment of CMT2C
Actio Biosciences recently announced that they have received both orphan drug designation and rare pediatric disease designation for ABS-0871, a TRPV4 inhibitor, for the treatment of TRPV4+ Charcot-Marie-Tooth disease subtype 2C (CMT2C) from the U.S. Food and Drug...

Augustine Therapeutics Raises $18.5 Million in a Series A First Closing
The Charcot-Marie-Tooth Research Foundation participated in the $18.5 million Series A funding round recently announced by Augustine Therapeutics. The financing was led by Asabys Partners, with the participation of Eli Lilly & Company, CMTRF, and current investors...

CMT Research Foundation Invests in ReviR Therapeutics Research to Pioneer Small Molecule Therapeutics for CMT1A
The CMT Research Foundation has formed a strategic partnership with ReviR Therapeutics to advance small molecule therapeutics that modulate the expression of the gene that causes CMT1A. ReviR Therapeutics is developing a class of drugs called small molecule splice...

NMD Pharma Receives FDA Clearance to Initiate Clinical Trial of NMD670 for CMT
NMD Pharma recently announced that it has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application to initiate a Phase 2 clinical trial of NMD670 in patients living with CMT types 1 and 2. NMD670 is a...

CMTRF at the 2024 American Society of Gene and Cell Therapy Conference
We are thrilled to see that two CMTRF-funded projects were selected to present their advancements on their CMT gene therapy projects at the ASGCT conference, the premier conference for emerging genetic therapies. Dr. Lindsay Wallace presented a poster on the CMT1A...

CMT Research Foundation Invests in Project to Test if a Commercially Available Drug is Effective Treatment for X-linked Charcot-Marie-Tooth Disease
The CMT Research Foundation (CMTRF), a non-profit focused solely on delivering treatments and cures for Charcot-Marie-Tooth disease (CMT), has invested in a project led by Dr. Charles Abrams, a Professor in the Department of Neurology and Rehabilitation at the...

CMT Research Update/NMD Pharma
NMD Pharma recently published data demonstrating that their drug candidate, NMD670, improves muscle function and neuromuscular transmission deficits in both animal models and patients. Dr. William David Arnold, a member of the CMTRF Scientific Advisory Board, who was...

Vanderbilt’s Charles Sanders Successfully Finds Molecules That Alter PMP22 Production or Cell Surface Trafficking; In Next Phase Will Test if They Can Improve CMT-like Problems in Schwann Cells
CMT1A is caused by a gene-copying event that results in the overproduction of the peripheral myelin protein 22 (PMP22) in Schwann cells. The excess PMP22 protein fails to traffic normally to the cell surface and instead collects inside the cells as clumps of proteins...
Address
4062 Peachtree Road
Suite A209
Atlanta, GA 30319
Phone Number
404.806.7180
Media Inquiries
© 2024 CMT Research Foundation | Privacy Policy
