The CMT Research Foundation has awarded nearly $100,000 to support a 13-month project to test a new therapeutic delivery system that may be capable of crossing the blood-nerve barrier and releasing therapeutic payloads to the Schwann cells and axons of the peripheral nervous system.
The CMT Research Foundation has awarded nearly $100,000 to support a 13-month project to test a new therapeutic delivery system that may be capable of crossing the blood-nerve barrier and releasing therapeutic payloads to the Schwann cells and axons of the peripheral nervous system.
The award was made to Kelly Langert, PhD in the Department of Molecular Pharmacology and Neuroscience at Loyola University Chicago’s Stritch School of Medicine and the Research and Development Service of the Edward Hines Jr. Veterans Affairs Hospital. Dr. Langert will be joined in the project by Virginie Mansuy-Aubert, PhD, an Associate Professor at Stritch. Dr. Charles Abrams, neurologist and Professor at the University of Illinois at Chicago (UIC), one of the world’s foremost experts on CMTX, and member of the CMT Research Foundation’s Scientific Advisory Board, will serve as a collaborator and advisor on the project.
Delivery of therapeutics to the peripheral nervous system is a fundamental problem in Charcot-Marie-Tooth disease and frequently results in otherwise promising therapies failing to advance, so much so that the CMT Research Foundation has deemed drug delivery as one of our top research priorities. Many substances in blood are toxic to nerve tissue, so the peripheral nerves are protected by a structure called the blood-nerve barrier, which prevents most molecules from moving out of the blood stream and into the tissues of the nerves. Typically, therapeutics cannot reach the cells of the peripheral nervous system at all or must be given in such high doses to reach the peripheral nervous system that it results in toxicity. Overcoming this problem is of major importance. One way to do this may be to package therapeutics in such a way that the blood-nerve barrier is “tricked” into allowing them access to the nerve tissue, much like we “trick” pets into taking a pill by wrapping it in cheese.
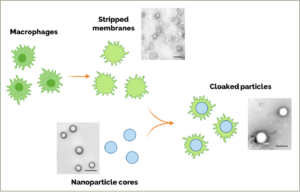
In addition to neurons and Schwann cells, the peripheral nerves also contain several other cell types, including macrophages, which can cross the blood-nerve barrier when the nerve is inflamed. Dr. Langert’s lab is developing a way to strip the outer membrane from macrophages and then use this membrane to “cloak” tiny nanoparticles that can carry a therapeutic payload. The idea behind this proposal is that inflamed peripheral nerves may allow passage of these “cloaked” nanoparticles through the blood-nerve barrier, where they can then release therapeutics locally in the nerve tissue. Moreover, these nanoparticles are composed of slow-release materials, potentially further enhancing their ability to minimize side effects.
“I am excited by the opportunity to test this new technology for application in CMT,” said Dr. Langert. “Advances in nanomedicine and our understanding of the active role that immune cells like macrophages play in the nerve are allowing us to approach treatment of peripheral nerves in ways never before possible.”
This kind of cutting-edge research fits squarely within the CMT Research Foundation’s mission to deliver treatments and cures for CMT in our lifetime. We look forward to keeping you updated on this project and all the research we fund.