News & Stories
See the latest news about CMT drug development and read stories from the CMT community that highlight why we must deliver treatments and cures during our lifetime.

NMD Pharma Publishes Findings Supporting the Role of ClC-1 Inhibition in Charcot-Marie-Tooth Disease
NMD Pharma announced that they have published new clinical and preclinical data that provides evidence that targeting neuromuscular junction deficits could potentially improve muscle function in CMT patients. The peer-reviewed paper titled “Neuromuscular Transmission...

Update on NMD Pharma Clinical Trial
NMD Pharma, a clinical-stage biotech company dedicated to developing novel and improved treatments for patients living with neuromuscular diseases, has announced that it has dosed the first Charcot-Marie-Tooth disease patient in its Phase 2a clinical trial of NMD670...
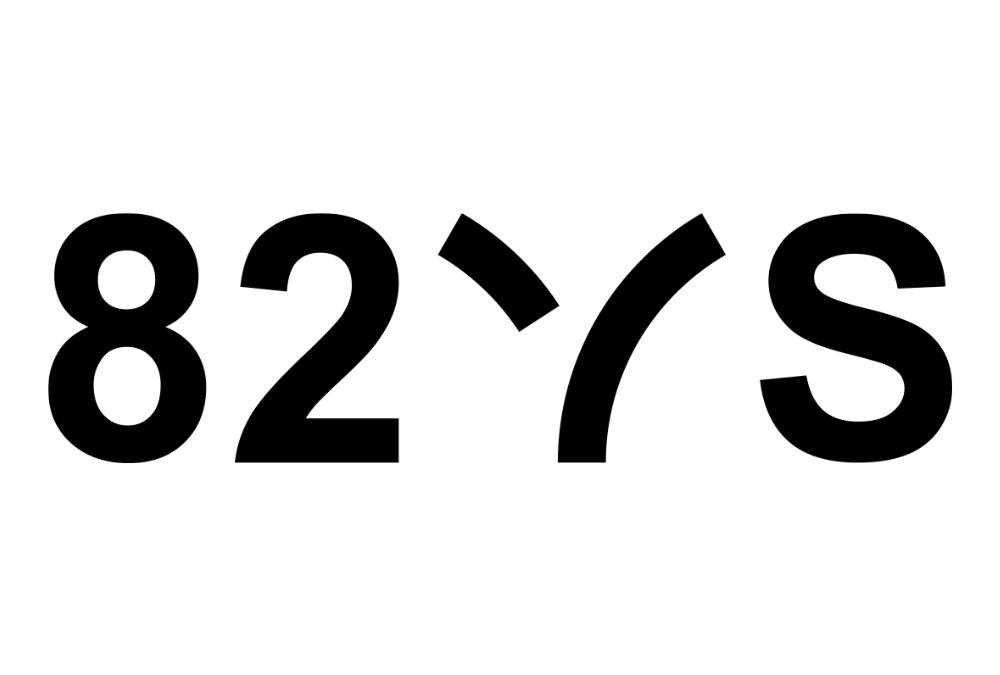
CMT Research Foundation Funds Project with Alloy Therapeutics’ 82VS to Test Novel RNA Therapeutics for Charcot-Marie-Tooth Disease
The CMT Research Foundation has invested over $500,000 in a program led by 82VS, the venture studio of Alloy Therapeutics, Inc. to discover and develop novel Antibody Oligonucleotide Conjugate (AOC) therapeutics for CMT. Oligonucleotide therapeutics offer promise for...

Dr. Alessandra Bolino Completes Project Testing PIKfyve Inhibitor Drug from AcuraStem in CMT4B1 models
Previous research from Dr. Alessandra Bolino demonstrated that inhibiting a protein called PIKfyve kinase could ameliorate some disease characteristics in CMT4B1 cells. In this project, Dr. Bolino partnered with AcuraStem to test one of their PIKfyve inhibitor drug...

Actio Biosciences Receives Both Orphan Drug and Rare Pediatric Disease Designations From the FDA For Treatment of CMT2C
Actio Biosciences recently announced that they have received both orphan drug designation and rare pediatric disease designation for ABS-0871, a TRPV4 inhibitor, for the treatment of TRPV4+ Charcot-Marie-Tooth disease subtype 2C (CMT2C) from the U.S. Food and Drug...

Armatus Bio Completes Pivotal Animal Study Demonstrating Delivery, Safety, and Activity of Their CMT1A Gene Therapy and is Now Preparing to Advance Project to Clinical Trials
Armatus Bio acquired the CMT1A gene therapy that was developed by Dr. Kleopas Kleopa and Dr. Scott Harper in a previous CMTRF-funded project to advance it further as a therapeutic. To demonstrate this therapy could be a safe and effective treatment for CMT1A, Armatus...

Augustine Therapeutics Raises $18.5 Million in a Series A First Closing
The Charcot-Marie-Tooth Research Foundation participated in the $18.5 million Series A funding round recently announced by Augustine Therapeutics. The financing was led by Asabys Partners, with the participation of Eli Lilly & Company, CMTRF, and current investors...

CMT Research Foundation Invests in ReviR Therapeutics Research to Pioneer Small Molecule Therapeutics for CMT1A
The CMT Research Foundation has formed a strategic partnership with ReviR Therapeutics to advance small molecule therapeutics that modulate the expression of the gene that causes CMT1A. ReviR Therapeutics is developing a class of drugs called small molecule splice...

NMD Pharma Receives FDA Clearance to Initiate Clinical Trial of NMD670 for CMT
NMD Pharma recently announced that it has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application to initiate a Phase 2 clinical trial of NMD670 in patients living with CMT types 1 and 2. NMD670 is a...

CMTRF at the 2024 American Society of Gene and Cell Therapy Conference
We are thrilled to see that two CMTRF-funded projects were selected to present their advancements on their CMT gene therapy projects at the ASGCT conference, the premier conference for emerging genetic therapies. Dr. Lindsay Wallace presented a poster on the CMT1A...
