News & Stories
See the latest news about CMT drug development and read stories from the CMT community that highlight why we must deliver treatments and cures during our lifetime.

CMT Research Foundation Funds Elpida Therapeutics to Launch Manufacturing of a Gene Therapy Drug for CMT4J
CMT Research Foundation announced they have funded Elpida Therapeutics to launch manufacturing of a gene therapy drug for Charcot-Marie-Tooth disease type 4J. The announcement came at the 2025 Global CMT Research Convention. “Our ELP-02 gene therapy is trial-ready,”...
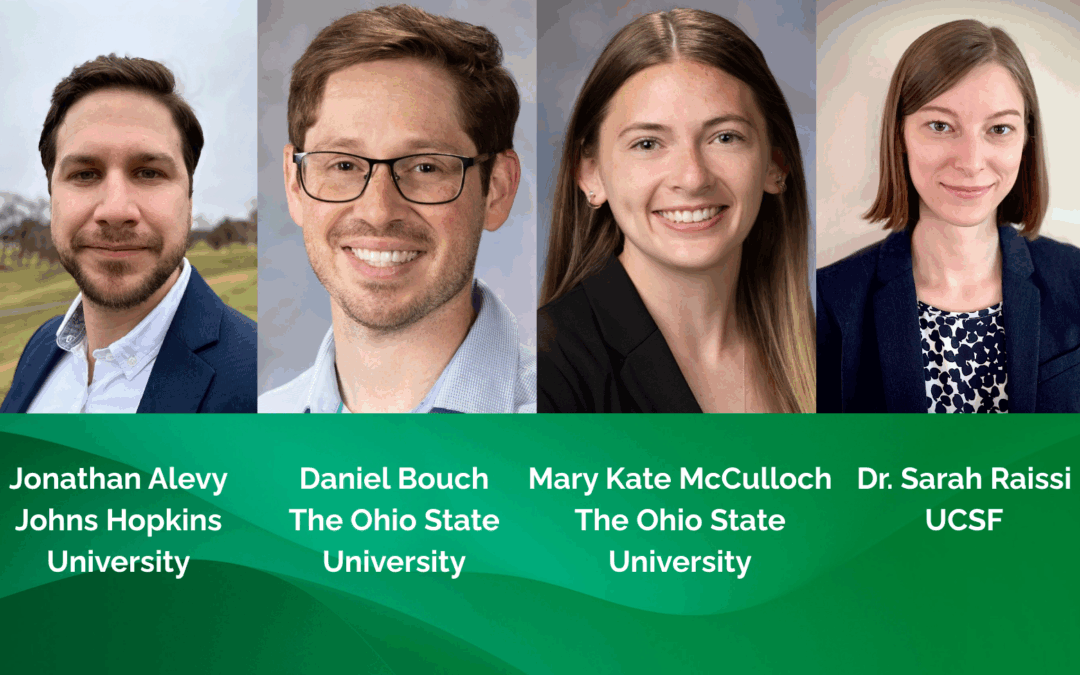
CMT Research Foundation Invests in the Next Generation of CMT Researchers
The CMT Research Foundation has awarded funding to four new research projects through its Emerging Researcher Award program, which provides small, high-impact grants to promising scientists in academia and industry. Spanning multiple Charcot-Marie-Tooth disease...

Applied Therapeutics to Meet With FDA on New Drug Application for the Treatment of CMT-SORD
Applied Therapeutics, who will be speaking at the 2025 Global CMT Research Convention, recently announced that they will be meeting with the U.S. Food and Drug Administration, during the third quarter of 2025, to discuss a potential New Drug Application for...

Dr. Charles Abrams Discovers That Inosine Treatment Leads to Benefits in a CMT1X Animal Model
Dr. Charles Abrams, Professor of Neurology and Rehabilitation at the University of Illinois, Chicago, has discovered that inosine could potentially reduce nerve inflammation and offer moderate functional benefits in preclinical animal models of Charcot-Marie-Tooth...

CMT Research Foundation Invests in XtRNA Bio to Develop Gene Therapy for CMT2D
CMT Research Foundation has invested in a research project with XtRNA Bio aimed to develop a new viral gene therapy for Charcot-Marie-Tooth disease type 2D. How GARS1 Mutations Drive CMT2D CMT2D is caused by mutations in the GARS1 gene, which encodes glycyl-tRNA...

Applied Therapeutics Presents Findings From Phase 2/3 Clinical Trial of Govorestat in CMT-SORD
Applied Therapeutics recently presented findings from their INSPIRE Phase 2/3 clinical trial of govorestat (AT-007) for the treatment of Sorbitol Dehydrogenase Deficiency, a subtype of Charcot-Marie-Tooth disease, at the Peripheral Nerve Society’s 2025 Annual Meeting...

CMT Research Foundation Invests in Asha Therapeutics to Test Novel Drug for CMT2A
CMT Research Foundation has invested in a research project with Asha Therapeutics to investigate the therapeutic potential of inhibiting SARM1 (Sterile alpha and TIR motif-containing protein 1) using their novel drug, ASHA-624, for the treatment of Charcot-Marie-Tooth...

Vanderbilt University Researchers Identify Promising Compounds Targeting PMP22 Protein Expression, Paving the Way for New Treatments for CMT1A, CMT1E and HNPP
Researchers at Vanderbilt University have made significant progress in the development of small molecules aimed at treating Charcot-Marie-Tooth disease types caused by mutations in the PMP22 gene, which include CMT1A, CMT1E and hereditary neuropathy with liability to...

Dr. Alessandra Bolino Unravels Complexities and Tests Treatment Strategies in CMT4B1
CMT4B1 is a severe form of Charcot-Marie-Tooth disease caused by mutations in the gene that makes Myotubularin-related protein 2 (MTMR2). Dr. Alessandra Bolino, PhD, discovered that mutations in this gene affect the production of fats, called phospholipids. Among...
82VS Progressing Ahead of Schedule in CMTRF-Funded Project to Improve Oligonucleotide Drug Delivery for CMT
Since launching their collaboration with CMT Research Foundation in November 2024, researchers at 82VS, the venture studio of Alloy Therapeutics, have made rapid progress and are ahead of schedule testing their novel technology focused on improving RNA therapeutic...
