News & Stories
See the latest news about CMT drug development and read stories from the CMT community that highlight why we must deliver treatments and cures during our lifetime.

CMTRF Earns Top Rating From Charity Navigator
The CMT Research Foundation has been evaluated for the first time by Charity Navigator, the nation’s largest charity evaluator, and earned a Four-Star Rating, the highest possible rating. Charity Navigator’s impartial, third-party evaluation validates CMTRF’s...
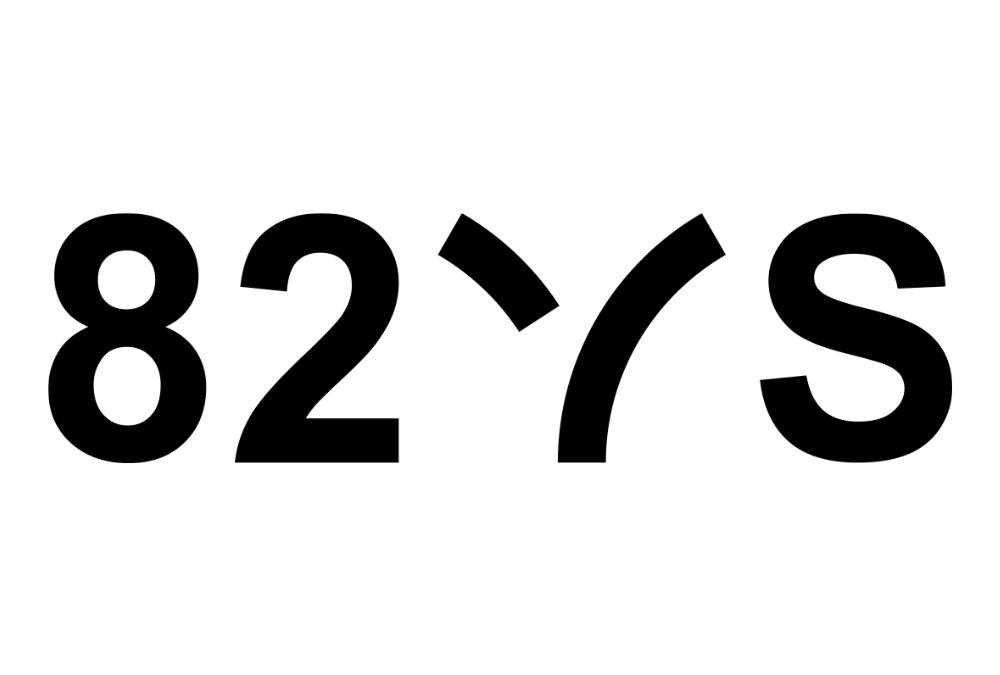
CMT Research Foundation Funds Project with Alloy Therapeutics’ 82VS to Test Novel RNA Therapeutics for Charcot-Marie-Tooth Disease
The CMT Research Foundation has invested over $500,000 in a program led by 82VS, the venture studio of Alloy Therapeutics, Inc. to discover and develop novel Antibody Oligonucleotide Conjugate (AOC) therapeutics for CMT. Oligonucleotide therapeutics offer promise for...

Dr. Alessandra Bolino Completes Project Testing PIKfyve Inhibitor Drug from AcuraStem in CMT4B1 models
Previous research from Dr. Alessandra Bolino demonstrated that inhibiting a protein called PIKfyve kinase could ameliorate some disease characteristics in CMT4B1 cells. In this project, Dr. Bolino partnered with AcuraStem to test one of their PIKfyve inhibitor drug...

Laura MacNeill Named New CEO of CMT Research Foundation
The CMT Research Foundation has hired Laura MacNeill as Chief Executive Officer, it was announced today by Foundation Board Chair Peter de Silva. Ms. MacNeill succeeds Cleary Simpson, CEO for the past three years, who becomes Vice Chair of the Board. “Ms. MacNeill has...

CMT Research Foundation Surpasses $10 Million Goal of ENDGAME Capital Campaign
The CMT Research Foundation, a non-profit focused solely on delivering treatments and cures for Charcot-Marie-Tooth disease announced over the weekend at its annual Global CMT Research Convention that it has surpassed its goal of raising $10 million to fund research...

Actio Biosciences Receives Both Orphan Drug and Rare Pediatric Disease Designations From the FDA For Treatment of CMT2C
Actio Biosciences recently announced that they have received both orphan drug designation and rare pediatric disease designation for ABS-0871, a TRPV4 inhibitor, for the treatment of TRPV4+ Charcot-Marie-Tooth disease subtype 2C (CMT2C) from the U.S. Food and Drug...

CMT Research Foundation Invests in Project at Nationwide Children’s Hospital to Develop Improved Gene Therapy Delivery Systems for Several Types of CMT
The CMT Research Foundation, a non-profit focused solely on delivering treatments and cures for Charcot-Marie-Tooth disease (CMT)*, has invested in a research project to develop new vehicles for delivering gene therapies more efficiently into peripheral nerves. This...

Armatus Bio Completes Pivotal Animal Study Demonstrating Delivery, Safety, and Activity of Their CMT1A Gene Therapy and is Now Preparing to Advance Project to Clinical Trials
Armatus Bio acquired the CMT1A gene therapy that was developed by Dr. Kleopas Kleopa and Dr. Scott Harper in a previous CMTRF-funded project to advance it further as a therapeutic. To demonstrate this therapy could be a safe and effective treatment for CMT1A, Armatus...

The CMT Research Foundation’s 2024 Global CMT Research Convention to Gather Renowned Scientific Experts and Patients to Discuss Status of Treatments and Cures for Charcot-Marie-Tooth Disease
The CMT Research Foundation recently announced most of the speakers at its upcoming 2024 Global CMT Research Convention to be held September 26-28, in Cambridge, MA. On Thursday, September 26, CMTRF will host a Young Researcher Innovation Forum for CMT Research....

CMT Research Foundation to Launch Grant Program to Support Young Investigators Working on CMT
The CMT Research Foundation announced that at its annual Global CMT Research Convention this September, it will host a Young Researcher Innovation Forum for CMT Research. The program will introduce the Young Researcher Innovation Grant and provide a platform to...
Address
4062 Peachtree Road
Suite A209
Atlanta, GA 30319
Phone Number
404.806.7180
Media Inquiries
© 2024 CMT Research Foundation | Privacy Policy
