News & Stories
See the latest news about CMT drug development and read stories from the CMT community that highlight why we must deliver treatments and cures during our lifetime.

ORYZON collaborates with the CMT Research Foundation in the US
CMTRF to provide funding for in vivo efficacy tests of Oryzon’s HDAC6 inhibitors in Charcot-Marie-Tooth preclinical model MADRID, SPAIN and Atlanta, July 26, 2022 Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company...

The CMT Research Foundation Invests Over $500,000 to Find a Single Gene Therapy for Possibly Every Form of CMT1B
CMT1B is one of the most common forms of CMT. In CMT1B, the part of the nervous system that is dysfunctional is the myelin sheath of the peripheral nerves. Created by cells called Schwann cells, myelin wraps around the parts of the nerves that facilitate rapid...

Project Testing PIKfyve Inhibitor in CMT4B1 Clears First Hurdles
CMTRF is happy to report that a CMTRF-funded project designed to test a PIKfyve inhibitor in CMT4B1, a particularly devastating form of the disease, has passed the first stage and is moving on to the next stage, a preclinical trial in CMT4B1 model mice. Before the...
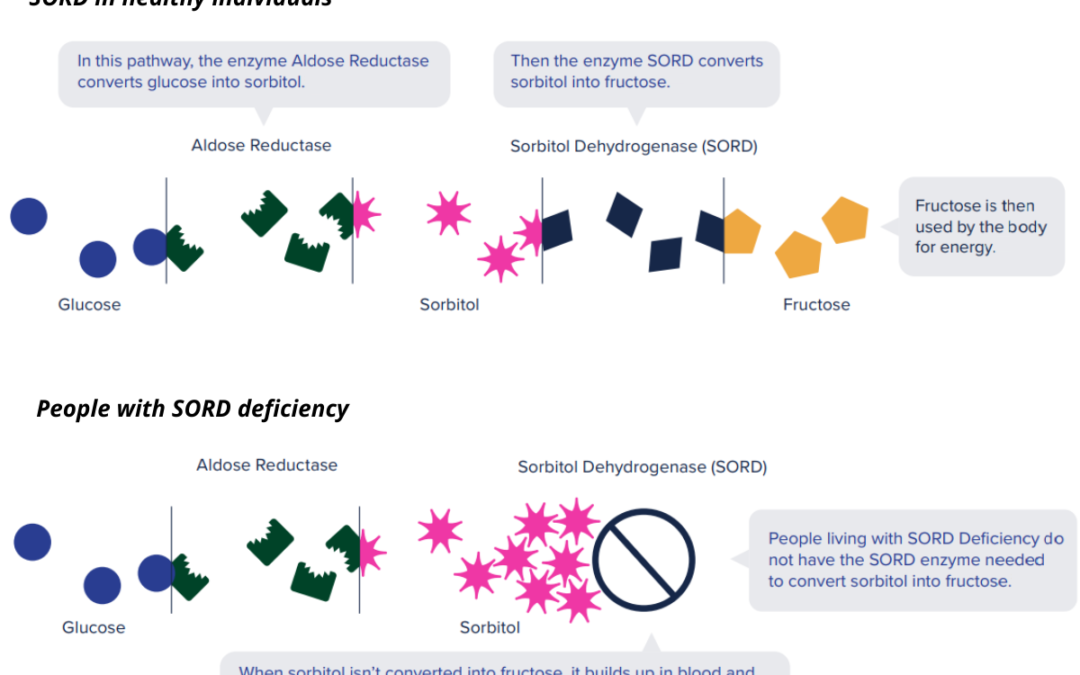
A New Trial for CMT – When Science Moves Quickly
By Keith Fargo, Ph.D., Chief Scientific Officer, CMT Research Foundation The pace of science can seem excruciatingly slow. It can take many years, or even decades, for new ideas to result in clinical trials. Every so often, though, the stars align and a new...
2022 Global CMT Research Convention—Introducing the Scientific Program Committee
As we get ready for the 2022 Global CMT Research Convention, one of the most important events is the empanelment of a Scientific Program Committee, or SPC. What does the SPC do? In general, an SPC assists in the development of the scientific content for a convention....

Shift Pharmaceuticals Completes CMT Research Foundation-Funded Project, Will Continue Development of Gene Therapy for CMT1A
The CMT Research Foundation is pleased to announce that Shift Pharmaceuticals (Shift) has successfully completed their CMT Research Foundation-funded RNA-based therapy project. Shift created and tested a library of novel molecules designed to reduce the expression of...
Family Affected by CMT1A Makes a Cure Their ‘Endgame’
Published by CMT News (slightly edited for clarity) It was an unexpected $1 million gift even for Kent and Michele Stahl. The Charcot-Marie-Tooth Research Foundation (CMTRF) was hosting a research convention in September when it announced Endgame, the organization’s...
Five Reasons Why We Think the End of CMT is in Sight
Nearly every instance of CMT is caused by the mutation of a single gene and most genetic causes of CMT have been identified. The era of genetic medicine has already begun. The FDA first approved genetic medicines in 2016/2017. This set the foundation for a precision...
The Power of Scientific Collaboration – New Biomarkers for CMT
At the CMT Research Foundation, we believe that more is achieved when people work together than when they work individually. This is especially true in biomedical research. Over the past few decades, a seismic shift has occurred in science with cooperation becoming...

Gene Therapy Project for CMT2E has Completed Milestone 1 Successfully
University of Missouri features CMT in a video about the project and the role of precision medicine to treat CMT. With the recent opening of their new NextGen Precision Health Institute, University of Missouri is quickly rising as a leader in precision medicine....
NEWSLETTER SIGNUP
Stay up to date on new CMT research, treatments and clinical trials
Address
4062 Peachtree Road
Suite A209
Atlanta, GA 30319
Phone Number
404.806.7180
Media Inquiries
© 2024 CMT Research Foundation | Privacy Policy
