By Keith Fargo, Ph.D., Chief Scientific Officer, CMT Research Foundation
The pace of science can seem excruciatingly slow. It can take many years, or even decades, for new ideas to result in clinical trials. Every so often, though, the stars align and a new discovery can quickly move into the clinical trial phase and the rapid pace can feel almost miraculous. The world of CMT is experiencing just such a blessing now. Only two years ago, researchers discovered a CMT-causing mutation in the SORD gene, and the drug company Applied Therapeutics has already launched a trial, called the INSPIRE study.
What kind of CMT are we talking about?
When possible, we refer to specific forms of CMT by their type or subtype numbering and lettering, particularly when the genetic cause is known. However, this form of CMT does not yet have a full moniker, since the discovery of the mutation is so recent. However, people with a SORD mutation have been diagnosed based on clinical symptoms and nerve conduction testing as having either a Type 2 CMT or distal hereditary motor neuropathy (dHMN). People with this mutation may have undergone previous genetic testing that did not return a genetic cause. This condition can also be called SORD deficiency.
What is SORD?
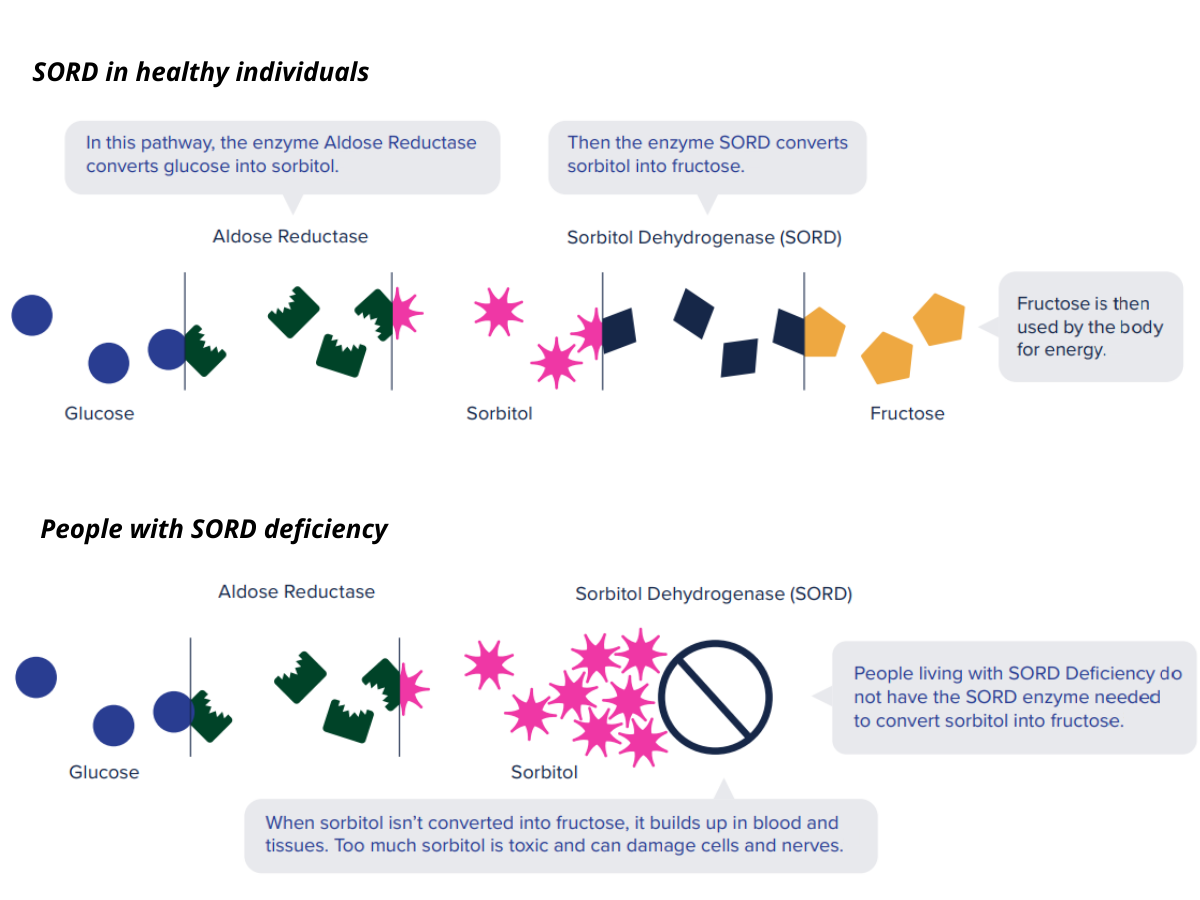
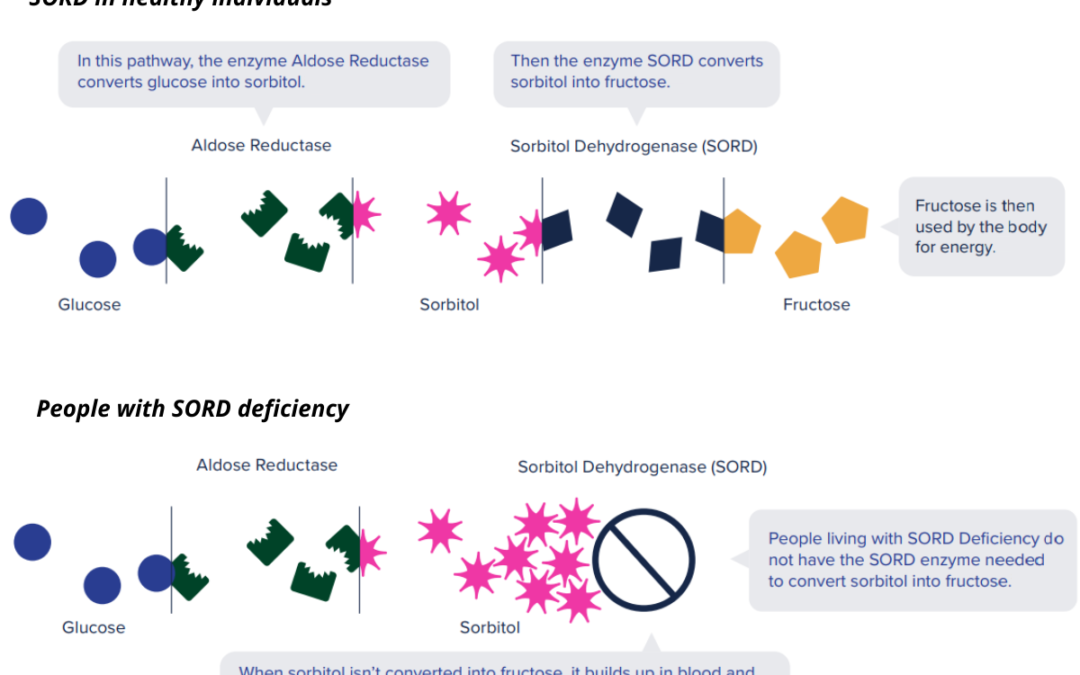
SORD stands for sorbitol dehydrogenase, referring both to the gene and the protein that it codes for. The SORD protein is an enzyme that processes sorbitol, a sugar that is naturally created in the body, by converting it to fructose. When a person’s SORD enzyme is deficient, however, sorbitol can build up to toxic levels in the body, resulting in neuropathy.
How did researchers move so quickly to a clinical trial?
Because the SORD enzyme is part of a metabolic pathway, researchers realized that there may be another way to prevent the toxic buildup of sorbitol. Instead of increasing the rate at which sorbitol is converted to fructose, it may be possible to reduce sorbitol levels by interfering with its production. In the body, the primary source of sorbitol is glucose, which is converted to sorbitol by another enzyme, called aldose reductase. By lowering the activity of aldose reductase, it may be possible to prevent the toxic buildup of sorbitol in the first place.
Luckily, the scientists at Applied Therapeutics were already developing aldose reductase inhibitors for potential use in diabetes, allowing them to quickly test the investigational medication in this form of CMT. The particular aldose reductase inhibitor to be used in the INSPIRE study is called AT-007. In an earlier trial, AT-007 demonstrated a tolerable safety profile, and people who took it had reduced levels of sorbitol in their bodies. AT-007 is taken by mouth once per day.
Who is potentially eligible for the study?
People between the ages of 16 and 55 with CMT or dHMN and no other major health problems may potentially be eligible for the INSPIRE study. People whose CMT is caused by another mutation are not eligible. Study participants will need to have confirmation of a SORD mutation, and genetic testing for the mutation will be provided by the study team free of charge for participants who may be eligible.
How do I find out more about the study?
If you or someone you know may be eligible for the INSPIRE study, there are several ways to learn more:
· Download the study brochure
· Visit the study website
· Email [email protected]

Momma suffered with CMT.
Any advances in this are so important. May you continue to discover as much as possible..thank God, for your research.