Success from the CMT Research Foundation’s Approach
DTx Pharma: Two years from initial funding to preparing for clinical trials
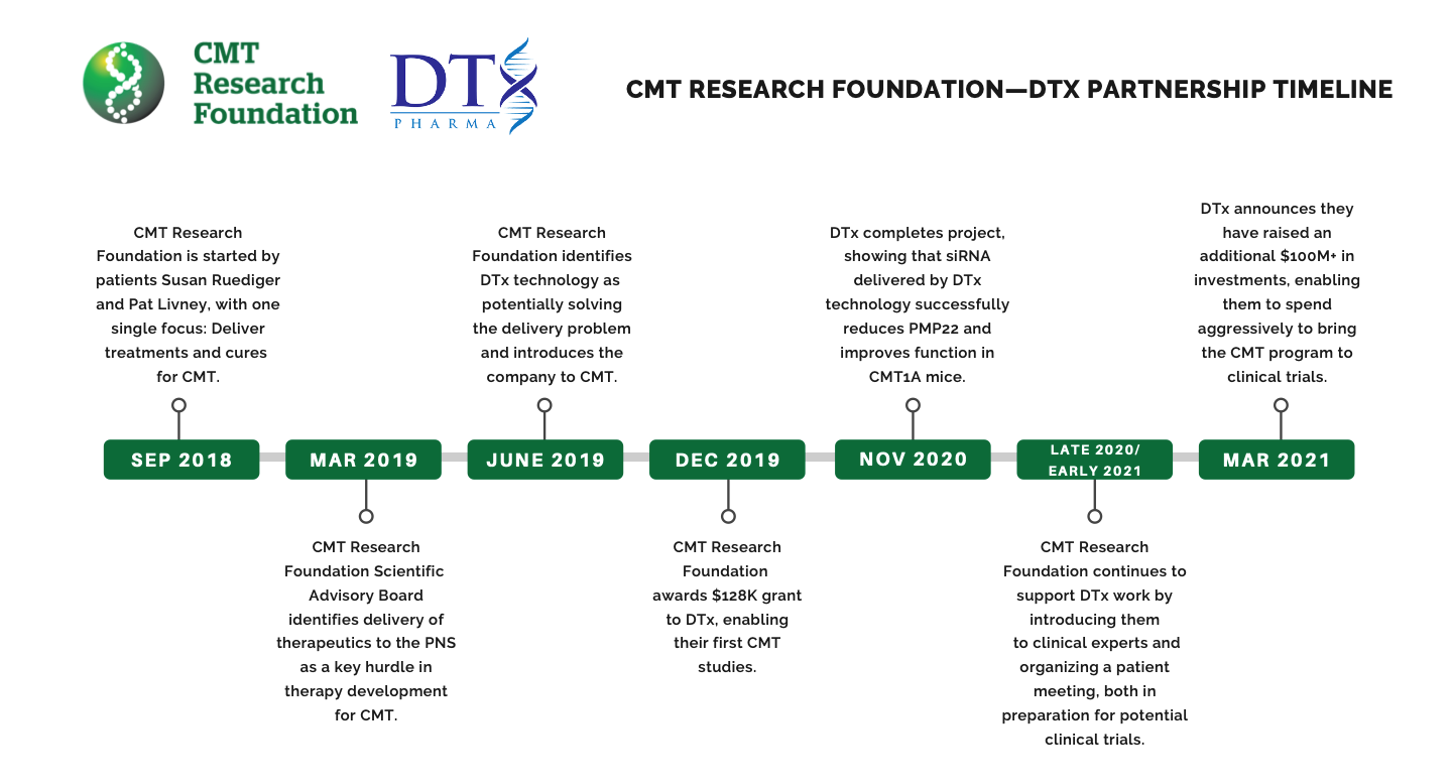
The CMT Research Foundation’s partnership with DTx Pharma provides striking evidence that our approach can multiply the impact of our grants, well beyond initial funding.
The CMT Research Foundation’s single focus on drug development demonstrates how a small amount of seed funding can power an idea into a therapy for patients. Without the CMT Research Foundation’s outreach to us and their early funding, DTx would not have started a CMT program.
Because the CMT Research Foundation approached us and provided us with the CMT expertise, we have been able to move quickly and realize the early success we’ve had. The CMT Research Foundation is smart, innovative, highly motivated and are simply great partners to have on this mission.
We understand the key barriers to developing a successful drug for CMT and have a strategy to attack them.
In 2019 we knew that a key barrier in drug development for CMT is the ability to deliver therapies throughout the peripheral nervous system.
We researched the pharmaceutical landscape and identified DTx Pharma as a company with similar therapies that might be adopted to CMT drug development. We introduced CMT to them, which hadn’t heard of previously. DTx leadership and the CMTRF Scientific Advisory Board, jointly designed a project with a unique approach to CMT1A drug delivery powered by an initial investment of $128,000.
The data from the initial project was promising enough to attract an additional $350,000 from the NIH to continue follow on studies. The results actually reversed CMT symptoms in mice. With this exceptional data, DTx was able to secure $100 million in private funding using talking points derived from meetings in which the Research Foundation team helped DTx understand the relevance of their work to the potential CMT market.
We have the commitment from DTx that, with continued success in the CMT1A project, they will invest enough capital into CMT1A to conduct clinical trials and apply for FDA approval. Our expectation is that by the end of 2021, DTx will have a drug ready for FDA consideration to move into clinical trials.
In two short years, CMT1A has promise for the first disease modifying drug that targets the cause of CMT. This started with a meeting in which the CMT Research Foundation team helped DTx understand the relevance of their work to a disease they had never heard of.
If this success is realized, we will have achieved an astonishing breakthrough—and the Foundation will share in the financial rewards, providing us with additional resources to aggressively reinvest in CMT research.
But we can’t be certain of DTx’s success, and we can’t wait to see. It is imperative that we move forward in pursuit of multiple possible treatments, since it is possible, even likely, that no single treatment will be ideal or will meet the needs of all those with CMT. We need to take further action now. CMT needs 100 more promising projects like DTx.
Read more about the DTx / CMT Research Foundation Partnership
Turning $128,000 into Millions to Deliver a Treatment for CMT.
https://cmtrf.org/innovation-in-cmt1a-science/
Mar 11, 2021 | CMT Research Updates, Gene Therapy, News, Research news
DTx Pharma Shows Continued Progress in Reducing PMP22 Levels in Animal Models of CMT1A
https://cmtrf.org/dtx-progress-update-cmt1a/
Nov 10, 2020 | CMT Research Updates, CMTRF Funded Research, Drug Development, Gene Therapy, Research news
CMT Research Foundation Partner DTx Pharma Wins Grant from National Institutes of Health for CMT1A Gene Therapy Program
Sep 22, 2020 | CMTRF Funded Research, Drug Development, Gene Therapy, Research news
DTx Pharma Achieves Second Milestone in Study to Advance Treatments for CMT1A
DTx Pharma Achieves Second Milestone in Study to Advance Treatments for CMT1A
Jun 26, 2020 | CMT Research Updates, CMTRF Funded Research, Drug Development, Research news | 1 comment
DTx Pharma Milestone 1 Completed
Apr 8, 2020 | CMTRF Funded Research, Research news
https://cmtrf.org/dtx-pharma-milestone-1-completed/
Partnership with DTx Pharma to optimize antisense oligonucleotides as a gene therapy for CMT1A
Dec 20, 2019 | CMT Research Updates, CMTRF Funded Research, Gene Therapy, Press Releases, Research news, Therapy Types | 1 comment
Partnership with DTx Pharma to optimize antisense oligonucleotides as a gene therapy for CMT1A
Research news
Find the latest news from around the world regarding CMT. Here you’ll find everything from drug development to clinical advances.
Research We Fund
Transparency is important to us. We provide detailed project descriptions about the research we support.
Apply for Funding
The CMTRF only funds projects that will lead toward approved drugs for CMT. To inquire about funding or to apply for funding, please contact Erich Fasnacht at [email protected].
Address
4062 Peachtree Road
Suite A209
Atlanta, GA 30319
Phone Number
404.806.7180
