What progress can patients and families expect to see in CMT research during 2021? What is the most promising research on the horizon? The CMT Research Foundation’s Chief Scientific Officer Keith Fargo, Ph.D., sat down with us to answer your most pressing questions about CMT research in the year ahead.

1. What excites you most about CMT research and drug development right now?
The sheer number of new approaches that are being taken in CMT research today. As a science-based organization, the CMT Research Foundation sees many new ideas in grant proposals and at scientific meetings before they become public information. There is a very exciting level of innovation in the field right now. CMT science is currently advancing across many fronts, which is exactly what we need to deliver treatments and cures as fast as possible.
2. What do you believe are the greatest areas of opportunity in advancing CMT research and discovery this year?
There are three areas where I see promising progress for the year ahead.
First, the United States Food and Drug Administration (FDA) released draft guidance in January 2021 for biotechnology and pharmaceutical companies that work on genetic therapies for neurodegenerative diseases such as CMT. This kind of guidance tends to speed and focus research.
Second, one of the most significant challenges in CMT drug development is ensuring therapeutics make it to the right cells in the right concentrations to have a beneficial effect without too many side effects. Researchers are quite creative, and we are seeing many innovative approaches to this problem in the grant proposals we receive. Consequently, we expect to see tangible progress against this challenge in the coming year.
Finally, recent advances in laboratory equipment are allowing researchers to measure some proteins at concentrations a thousand-fold lower than was possible just a few years ago. These advances are now being applied to CMT research and may potentially result in blood tests that can be used to more quickly and accurately measure the effectiveness of experimental drugs in future clinical trials for CMT.
READ NEXT: How to Measure Progress in CMT Research: 8 Ways to Know It’s Working
3. What are the CMT Research Foundation’s research priorities for 2021?
The CMT Research Foundation has one focus — to deliver treatments and cures for CMT. To that end, we will continue to emphasize finding and funding the best CMT research from around the globe. This year, we intend to expand our reach by introducing even more new researchers to CMT, funding more innovative research in genetic and traditional pharmaceutical therapies, and working with our research partners to overcome the most pressing challenges in drug development for CMT. You can read all about our research priorities here.
4. What can patients and families expect to see in 2021?
This year promises to be one of continuing advances in the fight against CMT. While we do not expect to see an FDA-approved therapy this year, several clinical trials are planned or currently underway, and more are set to launch this year. You can use our clinical trial finder to see if any may be right for you.
Additionally, the United States National Institutes of Health (NIH) has more than doubled the amount of CMT-related research they fund during the past three years, with $25 million committed in fiscal year 2020.
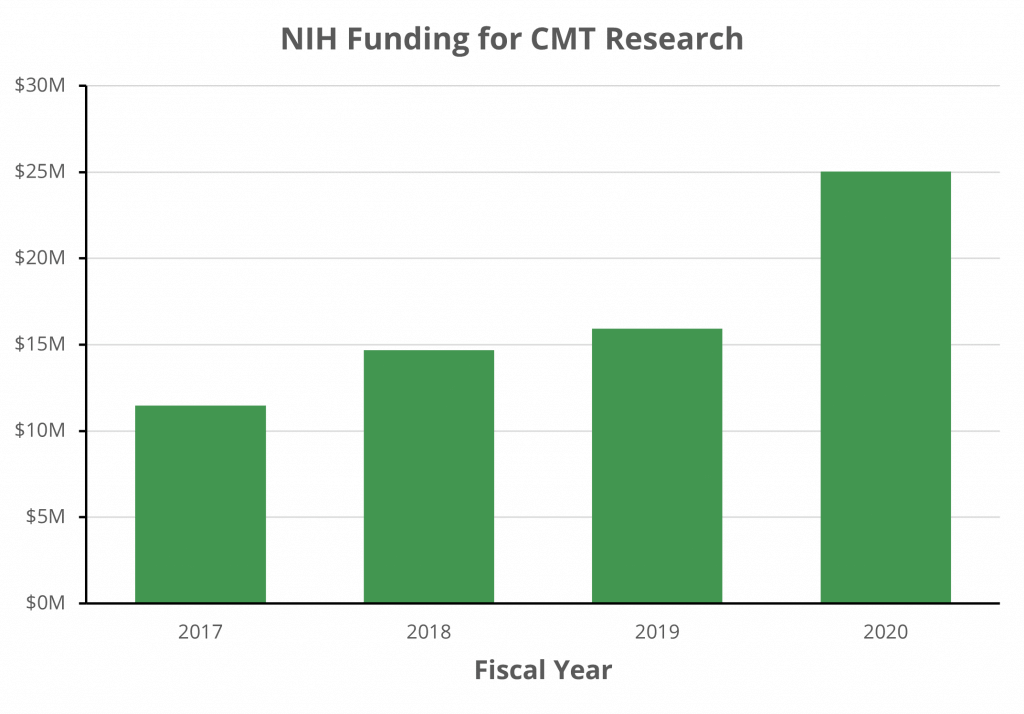
[Source: NIH RePORTER data]
5. What can the CMT community and supporters do this year that would be most helpful to advance progress?
We see more rapid advances in disease areas with involved patient communities.
Here are three ways you can help now:
- Contribute to the cause. Research costs money. The more we can invest in research, the faster we can deliver treatments and cures. If you are able to make a donation to the CMT Research Foundation, please consider doing so. It is the most important thing we can do to accelerate progress. The CMT Research Foundation also offers an ambassador program for those who want to take their personal fight to end CMT to the next level. We’d love for you to join the team!
- Use your voice. Sharing your CMT story and how the disease affects your life helps raise awareness about the disease, and awareness drives action. If you’d like to share your story with us, email us at [email protected].
- Participate in a clinical trial or natural history study. Clinical trials are critical to produce FDA-approved treatments and cures for CMT. And while natural history studies do not involve drug treatment, they are crucial for building information about the disease that makes future clinical trials faster and more effective. Each data point is one step closer to ending CMT.
READ NEXT: 5 Ways CMT Patients Can Take Action to Accelerate Drug Development
Stay Up-to-date on CMT Progress
To be the first to hear the latest update and progress in CMT research, sign up to join the CMT Research Foundation’s email list. You’ll receive exclusive updates and information on the latest research and other news that impacts patients and families with CMT. Join now.
